We've seen that blood clots are formed when fibrin molecules aggregate at the site of injury to form a fibrous clot. Fibrin is produced by cleaving the precursor fibrinogen. The enzyme that cuts the protein is a protease called thrombin [Blood Clotting: The Basics].
Thrombin is the active form of the protease enzyme. It is derived from a precursor called prothrombin and the production of thrombin at the site of injury requires an enzyme to cleave prothrombin. This enzyme is called prothrombinase. Like thrombin, prothrombinase is a protease only its substrate is prothrombin instead of fibrinogen.
Prothrombinase is a multisubunit enzyme composed of two different polypeptide chains. One of them is an activated form of Factor V (factor five) called FVa or just Va. The "a" signifies an activated form of a clotting factor. The other subunit of prothrombinase is an activated form of Factor X called FXa (activated factor ten). Xa plus Va together make the prothrombinase that cleaves prothrombin to make thrombin. This leads directly to blood clotting.
In order for this cascade to be initiated there has to be some trigger that leads to formation of prothrombinase (Xa + Va). This trigger has to be localized to the region where a blood vessel is damaged so that the blood clot forms at the right place. The initiation step is called the extrinsic activity.
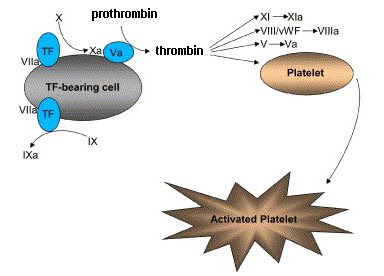
The cells lining blood vessels contain a membrane protein called tissue factor (TF). It is sometimes referred to by its old name Factor III. TF is normally masked but it becomes exposed when the cells are damaged. Factor VII (VII) binds to exposed TF to form a protease that cleaves Factor X to Xa. Xa plus Va then cleaves prothrombin to thrombin and thrombin activates a number of other factors that will enhance the clotting. There is always a little bit of Va circulating in the blood stream and it binds to TF-bearing cells when the membrane is exposed.
VII is one of the proteins containing a γ carboxyglutamyl residue. This is a modification that requires vitamin K [Vitamin K, Nobel Laureates: Dam and Doisy]. The γ carboxyglutamyl residue binds to calcium ions (Ca++). Calcium is an important cofactor in clot formation because there are several factors with γ-carboxyglutamyl resides. They must bind Ca++ because the postive charges allow the proteins to interact with negatively charged (anionic) surfaces such as those that are exposed when membranes are disrupted. The binding to anionic molecules explains why heparin (Monday's Molecule #20) inhibits clotting but we'll get to that another day.
The important activation steps that are catalyzed by thrombin are the conversion of Factor VIII to VIIIa and Factor IX to IXa. These contribute to the activation of platelets as we will see in the next posting. Factor VIII circulates in blood plasma as a complex with von Willebrand factor (vWF) but upon cleavage of VIII to VIIIa vWF dissociates. As you can see from the diagram, the first thrombin that is formed also cleaves Factor V to Va and this greatly increases the concentration of Va, which bind to the TF-bearing cells. This, in turn, leads to more prothrombinase being produced and more thrombin—an example of positive feedback.
The VIIa/TF complex also cleaves Factor IX to IXa. This serves to stimulate activated platelets.
Thrombin will also cleave some fibrinogen to fibrin initiating clot formation. However, the rate of fibrin formation that results from extrinsic activity is not sufficient to support the formation of a large clot. An additional step called intrinsic activity is needed to amplify the production of thrombin. This requires active platelets.
Active platelets look very different from the inactive forms. The active versions have a much more irregular shape and they have many extensions. It is the active platelets that aggregate to form a plug at the site of injury and the enhanced clotting activities take place on the platelet membrane surfaces.

[The clotting pathways are modified versions of figures from Wolberg, 2007]
No comments:
Post a Comment